CC(=O)O.OCC>[H+].[Cl-].OCC>CC(=O)OCC

Example
CC(=O)O.OCC>[H+].[Cl-].OCC>CC(=O)OCC


[CH2:1]=[CH:2][CH:3]=[CH:4][CH2:5][H:6]>>
[H:6][CH2:1][CH:2]=[CH:3][CH:4]=[CH2:5]
CC(=O)[OH]>>CC(=O)OCC
Matches any reaction with a reactant acid and a product ester. Includes all esterification reactions, plus any reaction which happens to have the appropriate functionality in its components.
C[C:2](=[O:1])[OH:1]>>C[C:2](=[O:1])OCC
Matches esterification reactions.
(C[C:2](=O)[OH].[OH:3]C)>>C[C:2](=O)[O:3]C
Matches any intra-molecular esterification reaction.
(C[C:2](=O)[OH]).([OH:3]C)>>C[C:2](=O)[O:3]C
Matches any inter-molecular esterification reaction.
| Example Reaction SMARTS: | ||
| Query: | Reaction: | Matches: |
C>>
| CC>>CN
| 2 |
>C>
| CC>>CN
| 0 |
>>C
| CC>>CN
| 1 |
C
| CC>>CN
| 3 |
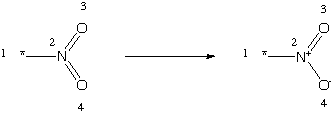
[*:1][N:2](=[O:3])=[O:4]>>[*:1][N+:2](=[O:3])[O-:4]
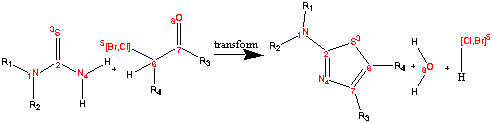
[N:1][C:2](=[S:3])[NH2:4]([H:99])[H:100].[Cl,Br:5]
[C:6]([H:101])[C:7]=[O:8]>>
[s:3]1[c:2]([N:1])[n:4][c:7][c:6]1.[H.99][O:8][H:100].[Cl,Br:5][H:101]
Contrast this with the "fragment marking approach"
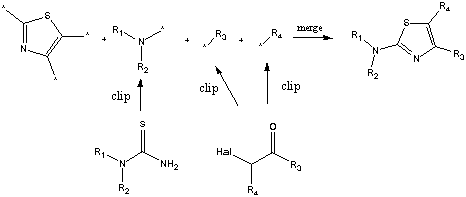
 Daylight Chemical Information Systems Inc.
Daylight Chemical Information Systems Inc.